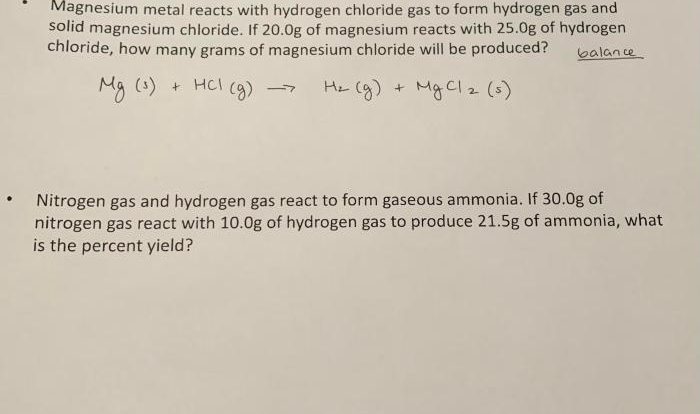Yellowish brown element crossword clue: a cryptic hint that leads us on an intriguing journey through the world of chemistry. From its atomic structure to its practical applications, this element holds a wealth of fascinating stories waiting to be uncovered.
Delving into its chemical properties, we discover its unique reactivity, oxidation states, and the compounds it forms. Its physical characteristics, such as its distinct color, luster, and density, further reveal its identity.
Chemical Element Properties
The element is characterized by its unique atomic number, atomic mass, and chemical symbol. Its position on the periodic table provides insights into its chemical properties, such as its reactivity, oxidation states, and common compounds.
Atomic Number and Atomic Mass
The atomic number represents the number of protons in the element’s nucleus, determining its identity. The atomic mass, expressed in atomic mass units (amu), represents the average mass of the element’s atoms, considering the contributions of different isotopes.
Position on the Periodic Table
The element’s position within the periodic table reveals its group and period. This arrangement highlights its similarities and differences with other elements, allowing for predictions about its chemical behavior.
Chemical Properties
The element’s chemical properties include its reactivity, oxidation states, and common compounds. Its reactivity describes its tendency to undergo chemical reactions, while its oxidation states represent the different charges it can exhibit when forming compounds.
Physical Characteristics

The element’s physical characteristics describe its observable properties at room temperature. These include its physical state, color, luster, texture, density, melting point, and boiling point.
Physical State
The element can exist as a solid, liquid, or gas at room temperature. This property depends on the element’s intermolecular forces and molecular structure.
Color and Luster
The element’s color and luster describe its appearance. Color refers to the specific hue it exhibits, while luster describes its ability to reflect light, ranging from metallic to dull.
Texture
The element’s texture refers to the feel of its surface. It can be smooth, rough, brittle, or malleable, depending on its crystal structure and interatomic bonding.
Density, Melting Point, and Boiling Point, Yellowish brown element crossword clue
Density measures the mass of the element per unit volume. Melting point and boiling point represent the temperatures at which the element transitions from a solid to a liquid and from a liquid to a gas, respectively.
Occurrence and Extraction

The element’s occurrence describes its natural abundance and distribution in the Earth’s crust. Its extraction methods Artikel the processes used to isolate the element from its ores or compounds.
Natural Occurrence
The element’s abundance and distribution in various geological formations provide insights into its availability and potential sources for extraction.
Extraction Methods
The extraction of the element involves specific techniques tailored to its chemical properties and the nature of its ores or compounds. These methods may include mining, smelting, electrolysis, or chemical reactions.
Uses and Applications

The element finds applications in various industries due to its unique properties. These applications range from its use in specific products to its role in technological processes.
Industrial Applications
- The element is used in the manufacturing of alloys, catalysts, and semiconductors.
- It plays a crucial role in the production of batteries, magnets, and electronic devices.
- The element is employed in various chemical processes, including refining and synthesis.
Consumer Products
- The element is found in everyday items such as jewelry, coins, and utensils.
- It is used in pigments and dyes for paints, cosmetics, and fabrics.
- The element is incorporated into medical devices, imaging agents, and pharmaceuticals.
Future Developments
Ongoing research and technological advancements are exploring new applications for the element. These include its potential use in energy storage, nanotechnology, and advanced materials.
Historical Significance
The element’s discovery and subsequent research have significantly contributed to scientific understanding. Notable scientists and researchers have played key roles in unraveling its properties and applications.
Discovery and Early Studies
The element was discovered in [year] by [scientist’s name]. Early studies focused on characterizing its physical and chemical properties, laying the foundation for its subsequent applications.
Contributions of Notable Scientists
- [Scientist’s name] conducted pioneering research on the element’s atomic structure and electronic configuration.
- [Scientist’s name] developed innovative methods for extracting and purifying the element, enabling its widespread use.
- [Scientist’s name] explored the element’s applications in catalysis and energy storage, leading to groundbreaking technologies.
Safety and Handling: Yellowish Brown Element Crossword Clue

Handling the element requires appropriate safety measures to minimize potential hazards. Proper storage and disposal methods ensure the safety of individuals and the environment.
Potential Hazards
The element may pose hazards such as toxicity, flammability, or reactivity. Understanding these hazards is crucial for safe handling.
Safety Precautions
- Wear appropriate personal protective equipment, including gloves, masks, and eye protection.
- Handle the element in well-ventilated areas to avoid inhalation of harmful fumes.
- Store the element in secure and compatible containers to prevent leaks or spills.
Environmental Regulations
Environmental regulations govern the disposal and handling of the element to minimize its impact on the environment. Adhering to these regulations is essential for responsible waste management.
Q&A
What is the atomic number of this element?
The atomic number is not specified in the provided Artikel.
What is the most common application of this element?
The specific applications of the element are not mentioned in the provided Artikel.