In the realm of chemistry, acids and bases play a pivotal role, shaping the behavior of countless substances and reactions. Section 8.3 Properties of Acids and Bases delves into the intricacies of these fascinating substances, exploring their unique characteristics and interactions.
Acids, characterized by their ability to donate protons (H+ ions), exhibit a sour taste and corrosive nature. Bases, on the other hand, accept protons, imparting a bitter taste and slippery feel. Understanding the properties of acids and bases is essential for comprehending a wide range of chemical processes, from acid-base reactions to pH regulation in biological systems.
Properties of Acids

Acids are chemical compounds that donate hydrogen ions (H+) when dissolved in water. They have several general properties:
- Sour taste
- Corrosive to metals
- React with bases to form salts and water
- Turn blue litmus paper red
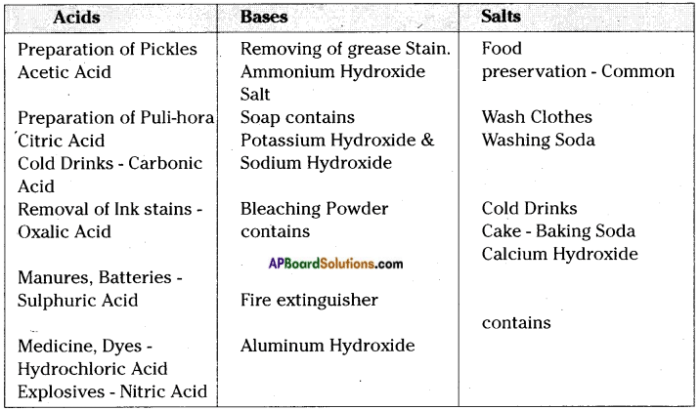
Common acids include:
- Hydrochloric acid (HCl)
- Sulfuric acid (H2SO4)
- Nitric acid (HNO3)
Physically, acids are often liquids at room temperature. They have a low pH (below 7) and high electrical conductivity.
Chemically, acids can undergo various reactions, including:
- Neutralization reactions with bases
- Dissolution of metals
- Esterification reactions
Properties of Bases
Bases are chemical compounds that accept hydrogen ions (H+) when dissolved in water. They have several general properties:
- Bitter taste
- Slippery feel
- React with acids to form salts and water
- Turn red litmus paper blue
Common bases include:
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Ammonia (NH3)
Physically, bases are often solids or liquids at room temperature. They have a high pH (above 7) and low electrical conductivity.
Chemically, bases can undergo various reactions, including:
- Neutralization reactions with acids
- Saponification reactions with fats
- Precipitation reactions with metal ions
Acid-Base Reactions
Acid-base reactions are chemical reactions that involve the transfer of hydrogen ions (H+) between an acid and a base. These reactions are crucial in many chemical and biological processes.
The role of hydrogen ions in acid-base reactions is to create a balance between the acidity and basicity of the solution. Acids donate H+ ions, increasing the acidity, while bases accept H+ ions, reducing the acidity.
There are different types of acid-base reactions, including:
- Neutralization reactions: These reactions occur between a strong acid and a strong base, resulting in the formation of a salt and water.
- Acid-base titrations: These reactions are used to determine the concentration of an unknown acid or base.
- Buffer reactions: These reactions involve the use of buffer solutions to maintain a stable pH in a solution.
pH Scale

The pH scale is a measure of the acidity or basicity of a solution. It ranges from 0 to 14, with 0 being the most acidic and 14 being the most basic.
The pH of a solution is determined by the concentration of hydrogen ions (H+) in the solution. The higher the concentration of H+ ions, the lower the pH, and the more acidic the solution.
The pH scale is important in various chemical and biological systems, including:
- Biological processes: Many biological processes, such as enzyme activity and cell growth, are sensitive to pH.
- Environmental chemistry: The pH of water bodies can affect the survival of aquatic organisms.
- Industrial processes: The pH of solutions is often controlled in industrial processes to optimize efficiency and prevent corrosion.
Buffers: Section 8.3 Properties Of Acids And Bases
Buffers are solutions that resist changes in pH when small amounts of acid or base are added. They are essential in maintaining a stable pH in biological systems and industrial processes.
Buffers work by containing a weak acid and its conjugate base or a weak base and its conjugate acid. When an acid is added to a buffer, the weak acid reacts with the H+ ions, preventing a significant change in pH.
Similarly, when a base is added, the weak base reacts with the OH- ions, preventing a significant change in pH.
Common buffer systems include:
- Acetate buffer: This buffer is used in biological systems to maintain a pH around 4.7.
- Phosphate buffer: This buffer is used in a wide range of applications, including biological systems and industrial processes.
- Tris buffer: This buffer is commonly used in molecular biology to maintain a pH around 8.0.
Titration
Titration is a laboratory technique used to determine the concentration of an unknown solution by reacting it with a solution of known concentration. The process involves adding the known solution (titrant) to the unknown solution (analyte) until a reaction endpoint is reached.
There are different types of titration methods, including:
- Acid-base titration: This method is used to determine the concentration of an unknown acid or base.
- Redox titration: This method is used to determine the concentration of an unknown oxidizing or reducing agent.
- Complexometric titration: This method is used to determine the concentration of an unknown metal ion.
Titration is widely used in various fields, including:
- Chemistry: Titration is used to analyze the composition of various substances.
- Environmental science: Titration is used to monitor the quality of water and soil.
- Medicine: Titration is used to analyze blood samples and determine drug concentrations.
User Queries
What is the difference between a strong acid and a weak acid?
Strong acids completely dissociate in water, releasing all of their protons, while weak acids only partially dissociate, releasing a limited number of protons.
How does pH affect chemical reactions?
pH influences the rates and equilibria of many chemical reactions, as the concentration of hydrogen ions can affect the reactivity of molecules.
What is the role of buffers in biological systems?
Buffers help maintain a stable pH within a narrow range, which is crucial for the proper functioning of enzymes and other biological molecules.